Avogadro's number. One mole contains 6 x 10 to the power 23 particles. How to find number of particles. Number of given moles x 6.0x10²³ particles/mol. for Amedeo Avogadro, number of particles contained in one mole of any substance; it is equal to 602,252,000,000,000,000,000,000, or in scientific notation, 6.0225210 23. For example, 12.011 grams of carbon (one mole of carbon) contains 6.0225210 23 carbon atoms, and 180.16 grams of glucose, C 6 H 12 O 6, contains 6.0225210 23 molecules of. Another property of Avogadro’s number is that the mass of one mole of a substance is equal to that substance’s molecular weight.
Question: Avogadro's Number Is Equal To The Number Of O Atoms In One-half Mole Of CO2 O Atoms In One Mole Of N2 O Molecules In One-half Mole Of N2 Atoms In 12 Grams Of 12C. This problem has been solved! Show transcribed image text. Expert Answer 100% (1 rating). The words for the Avogadro law are:equal volumes of all gases, at the same temperature and pressure, have the same number of molecules How many moles of atoms are in 6.0221023 atoms Ne?
[for Amedeo Avogadro], number of particles contained in one mole of any substance; it is equal to 602,252,000,000,000,000,000,000, or in scientific notation, 6.02252×1023. For example, 12.011 grams of carbon (one mole of carbon) contains 6.02252×1023 carbon atoms, and 180.16 grams of glucose, C6H12O6Avogadro's Number Practice
, contains 6.02252×1023 molecules of glucose. Avogadro's number is determined by calculating the spacing of the atoms in a crystalline solid through X-ray methods and combining this data with the measured volume of one mole of the solid to obtain the number of molecules per molar volume.
molecules of glucose. Avogadro's number is determined by calculating the spacing of the atoms in a crystalline solid through X-ray methods and combining this data with the measured volume of one mole of the solid to obtain the number of molecules per molar volume. The Columbia Electronic Encyclopedia, 6th ed. Copyright © 2012, Columbia University Press. All rights reserved.
See more Encyclopedia articles on: Chemistry: General
Avogadro's number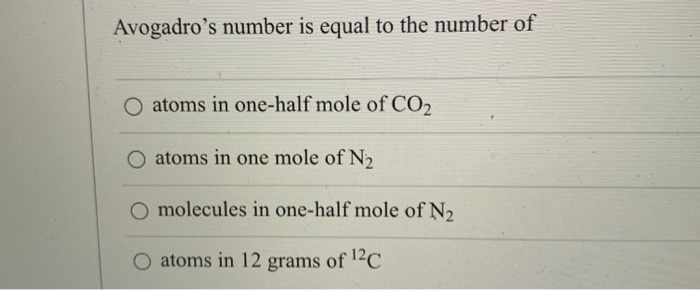 23 molecules of glucose. Avogadro's number is determined by calculating the spacing of the atoms in a crystalline solid through X-ray methods and combining this data with the measured volume of one mole of the solid to obtain the number of molecules per molar volume.
23 molecules of glucose. Avogadro's number is determined by calculating the spacing of the atoms in a crystalline solid through X-ray methods and combining this data with the measured volume of one mole of the solid to obtain the number of molecules per molar volume. The Columbia Electronic Encyclopedia, 6th ed. Copyright © 2012, Columbia University Press. All rights reserved.
How To Use Avogadro's Number
See more Encyclopedia articles on: Chemistry: General
